A decade since it’s discovery, a first-in-class, novel targeted immunotherapy developed at Duke Cancer Institute, has been brought to a phase 1b trial for patients with advanced-stage non-small cell lung cancer who have exhausted other therapeutic options.
The trial opened for recruitment through the Thoracic Oncology Program at DCI beginning in June and is still recruiting patients.
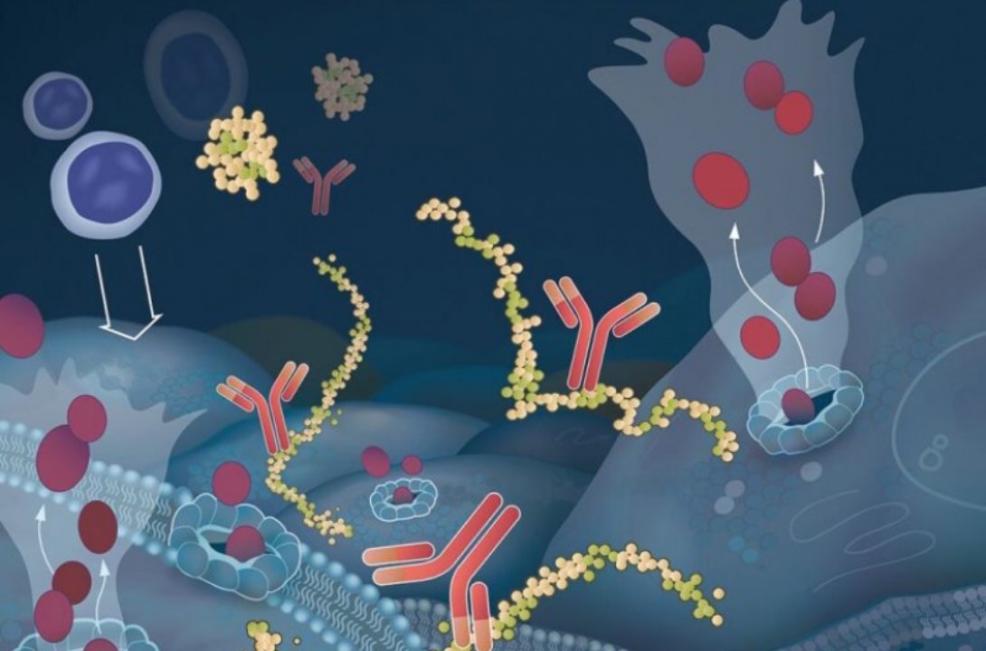
“We've got a really interesting homegrown drug here that nobody else is offering. It’s the first completely human-derived antibody developed as an anti-cancer therapy, and is very different from other immunotherapy approaches,” said Edward (Ned) F. Patz, Jr., MD, the CEO of Grid Therapeutics, LLC, the biotechnology company that developed the drug. “We believe this drug will be effective in many solid tumors and we're expecting it to have very little toxicity and side effects.”
To say that Patz, a Duke Cancer Institute clinical radiologist and professor in the departments of Radiology, Pharmacology and Cancer Biology, and Pathology, is “enthusiastic” about this latest DCI bench-to-bedside advance, his “baby,” is an understatement.
“I’m delighted with this program,” said Patz. “We came up with this here at Duke. It was a theoretical concept. We tried it in the lab. We figured it out. We’ve moved it forward. We (the field) are not doing very well with lung cancer. We need better drugs out there.”
Non-small cell lung cancer (NSCLC) accounts for about 85% of lung cancer cases, and the majority of patients have advanced stage disease at diagnosis. There’s a great need for new NSCLC therapies that work better and are less toxic.
“Lung cancer remains the number one cancer killer in the US. Too frequently our older, standard treatments will not work for patients and they carry many side effects,” said Jeffrey Clarke, MD, principal investigator on the trial and associate co-director, Cell Based Immunotherapy, at the DCI Center for Cancer Immunotherapy. “Fortunately, we have seen dramatic changes in recent years in the treatment landscape for lung cancer with new immune and targeted therapies. We still have a long way to go though, and we need to continue adding to our armamentarium in the fight against lung cancer. Our hope is that exciting drugs like GT103 result in improved side effect profile and increase survival for patients and their families.”