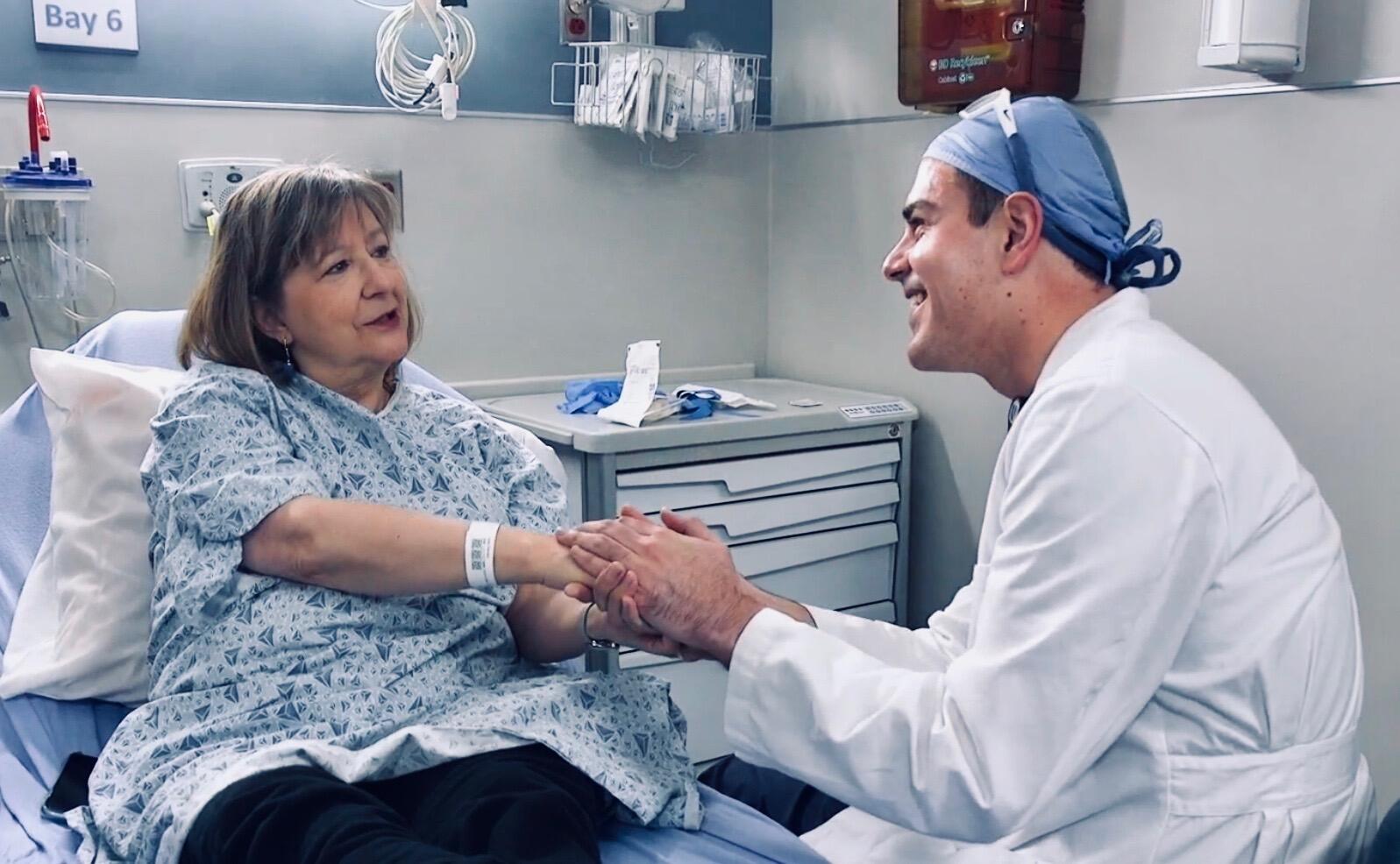
Pam Kohl, 68, has been living with metastatic breast cancer for more than three years.
Kohl, who’s well-known locally for her longtime leadership of the N.C. Triangle to the Coast Affiliate of the Susan G. Komen Foundation, was first diagnosed with stage 1 breast cancer in 2009.
After a lumpectomy, radiation, and five years of endocrine therapy, she was declared cancer-free.
But two years on, in Oct. 2016, a new tumor in the same breast was discovered. Following a mastectomy, PET scan and biopsy, she learned, on January 31, 2017, that the new cancer had already metastasized to two lymph nodes near her lungs.
Already taking endocrine therapy for the recurrence, she was then prescribed a relatively new oral targeted chemo therapy (Ibrance).
Kohl’s DCI oncologist and Komen Scholar Kimberly Blackwell, MD, explained that the Ibrance could potentially slow the progression of her cancer for the next 18 to 26 months. Essentially, she was given about two years to live — a day she'll never forget.
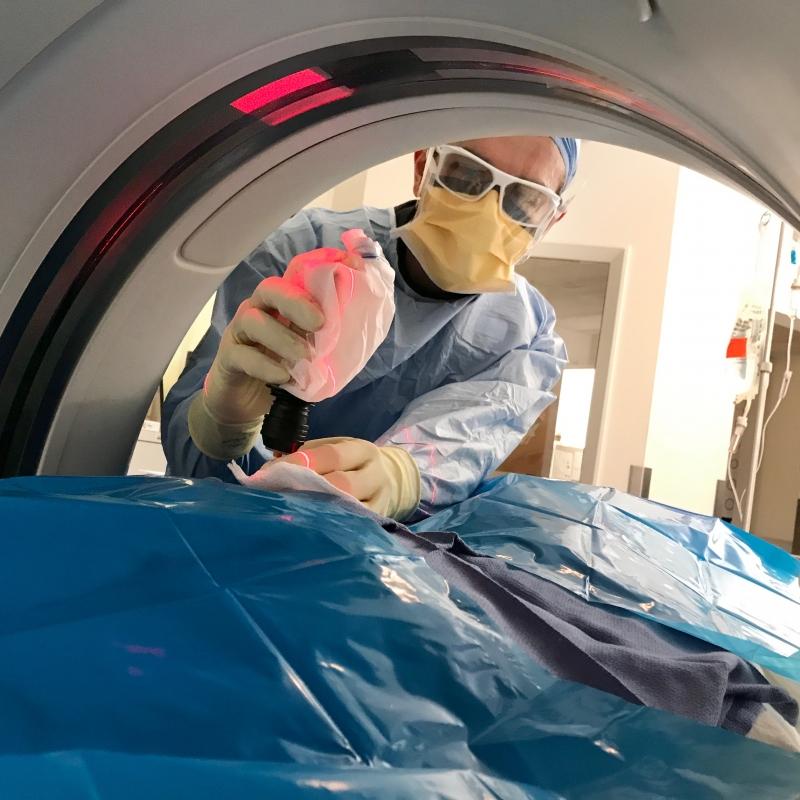
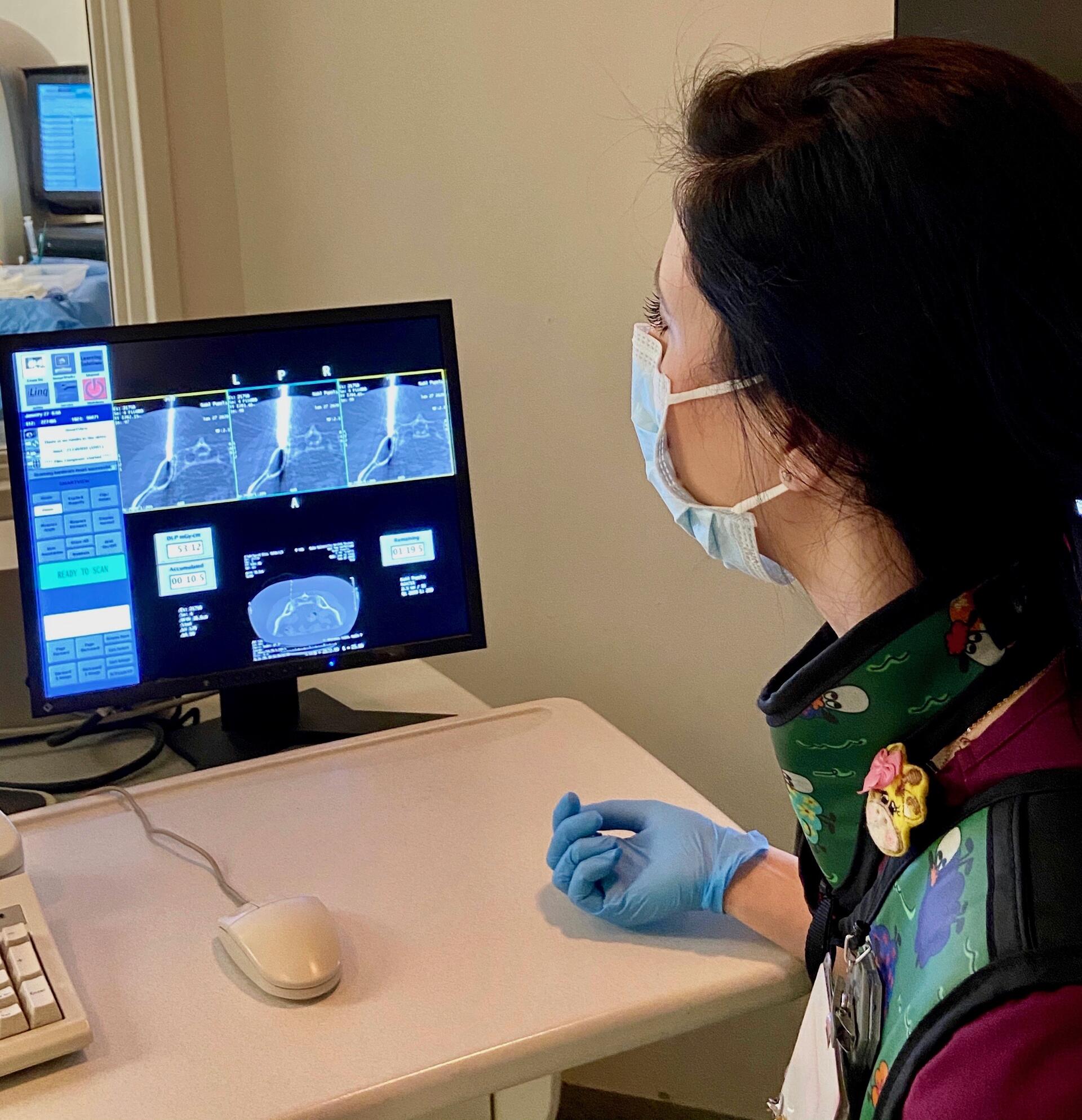
Provided the two tumors remained stable and no new tumors appeared in a year’s time, the plan was, Blackwell said, “to radiate the hell out of them.” When that year was up, in early 2018, Blackwell had left Duke, and Kohl was now under the care of breast oncologist Jeremy Force, DO, and radiation oncologist Rachel Blitzblau, MD, PhD.
“It was a slow, not-real-smart cancer, so we decided “Let’s go get ‘em,”” shared Kohl, confident in her treatment team.
The radiation worked. For about another year, with her cancer stable, she went about her business — educating the community about breast cancer prevention and treatment and raising funds for research. She was living well. Then, a setback. Over the 2018 holidays, a tumor emerged in her left hip.