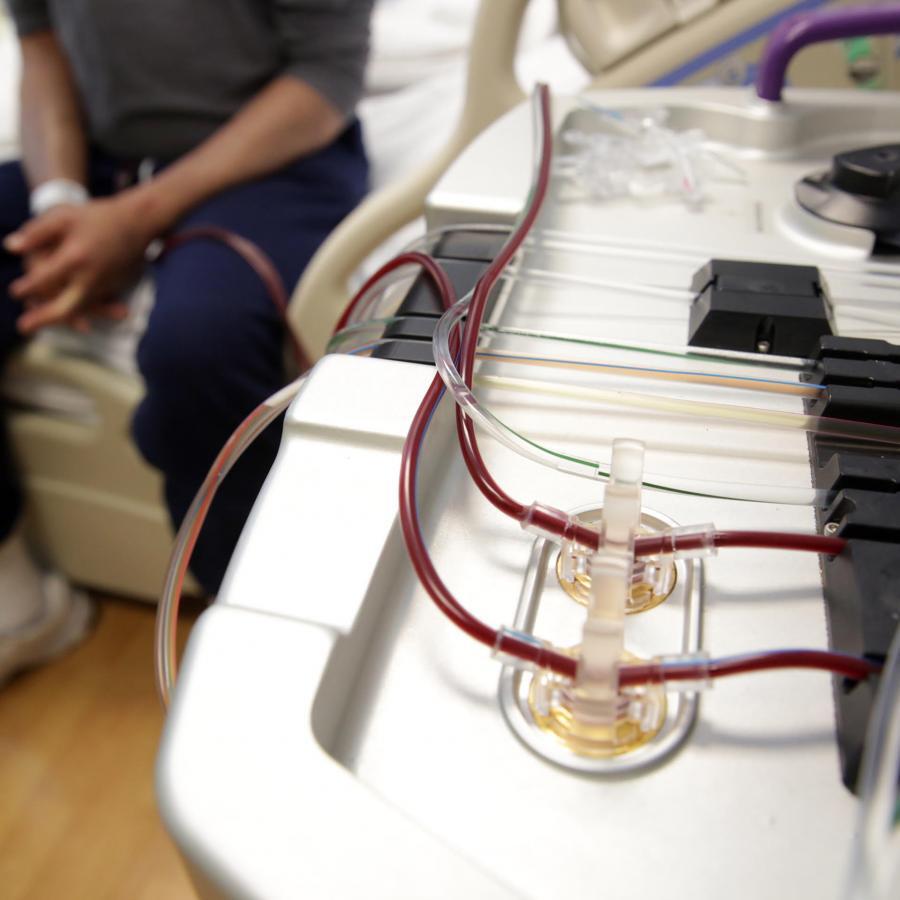
In the summer of 2016, three-time leukemia patient Connor McMahon, then 15, enrolled in a clinical trial at Duke of a novel immunotherapy using his own disease-fighting T-cells. While there was a significant risk of life-threatening complications, the Atlanta teen — under the care of Duke pediatric bone marrow transplant specialists Tim Driscoll, MD, and Paul Martin, MD —came through the trial with flying colors and was declared cancer free.
In sworn testimony before a U.S. Food and Drug Administration (FDA) panel the following summer, Connor’s father Don McMahon urged the regulatory body to approve the customized investigational therapy Connor received, a chimeric antigen receptor (CAR) T-cell therapy called Kymriah (tisagenlecleucel). Weeks later, Kymriah became the first commercially available gene therapy in the U.S. when it received accelerated approval from the FDA to treat children and young adult patients (through age 25) with relapsed/refractory B-cell acute lymphoblastic leukemia.
Natural human T-cells, which help orchestrate the body’s immune response, are often fooled by cancer’s disguises. CAR T-cell therapy gives them the boost they need. Unlike a pharmaceutical with a defined chemical formulation, CAR T-cell therapy is made from a patient’s own T-cells (a type of white blood cell called lymphocytes), which are re-engineered in a lab to produce proteins on their surface called chimeric antigen receptors (CARs). These CARs enable the T-cells, once infused back into the body, to recognize and bind to a specific overexpressed antigen (either CD19 or BCMA) on the surface of cancer cells that’s driving their out-of-control growth. The CARs bind with the antigens — like keys into a lock —deactivating those cancer cells.